Calcium chloride

| |

| |
| Names | |
|---|---|
| IUPAC name
Calcium chloride
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.030.115 |
| EC Number |
|
| E number | E509 (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CaCl2 | |
| Molar mass | 110.98 g·mol−1 |
| Appearance | White hygroscopic powder |
| Odor | Odorless |
| Density |
|
| Melting point | 772–775 °C (1,422–1,427 °F; 1,045–1,048 K) anhydrous[5] 260 °C (500 °F; 533 K) monohydrate, decomposes 175 °C (347 °F; 448 K) dihydrate, decomposes 45.5 °C (113.9 °F; 318.6 K) tetrahydrate, decomposes[5] 30 °C (86 °F; 303 K) hexahydrate, decomposes[1] |
| Boiling point | 1,935 °C (3,515 °F; 2,208 K) anhydrous[1] |
| Anhydrous: 74.5 g/100 mL (20 °C)[2] Hexahydrate: 49.4 g/100 mL (−25 °C) 59.5 g/100 mL (0 °C) 65 g/100 mL (10 °C) 81.1 g/100 mL (25 °C)[1] 102.2 g/100 mL (30.2 °C) α-Tetrahydrate: 90.8 g/100 mL (20 °C) 114.4 g/100 mL (40 °C) Dihydrate: 134.5 g/100 mL (60 °C) 152.4 g/100 mL (100 °C)[3] | |
| Solubility |
|
| Solubility in ethanol |
|
| Solubility in methanol |
|
| Solubility in acetone | 0.1 g/kg (20 °C)[4] |
| Solubility in pyridine | 16.6 g/kg[4] |
| Acidity (pKa) |
|
| −5.47·10−5 cm3/mol[1] | |
Refractive index (nD)
|
1.52 |
| Viscosity |
|
| Structure | |
| |
| |
| |
a = 6.259 Å, b = 6.444 Å, c = 4.17 Å (anhydrous, 17 °C)[6] α = 90°, β = 90°, γ = 90°
| |
| Octahedral at Ca2+ centres (anhydrous) | |
| Thermochemistry | |
Heat capacity (C)
|
|
Std molar
entropy (S⦵298) |
108.4 J/(mol·K)[1][5] |
Std enthalpy of
formation (ΔfH⦵298) |
|
Gibbs free energy (ΔfG⦵)
|
−748.81 kJ/mol[1][5] |
| Pharmacology | |
| A12AA07 (WHO) B05XA07 (WHO), G04BA03 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |
 [7] [7]
| |
| Warning | |
| H319[7] | |
| P305+P351+P338[7] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,000-1,400 mg/kg (rats, oral)[8] |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Calcium chloride is commonly encountered as a hydrated solid with generic formula CaCl2·nH2O, where n = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is hygroscopic and deliquescent, it is used as a desiccant.[10]
History
[edit]Calcium chloride was apparently discovered in the 15th century but wasn't studied properly until the 18th century.[11] It was historically called "fixed sal ammoniac" (Latin: sal ammoniacum fixum[12]) because it was synthesized during the distillation of ammonium chloride with lime and was nonvolatile (while the former appeared to sublime); in more modern times (18th-19th cc.) it was called "muriate of lime" (Latin: murias calcis, calcaria muriatica[12]).[13]
Uses
[edit]De-icing and freezing-point depression
[edit]
By depressing the freezing point of water, calcium chloride is used to prevent ice formation and is used to de-ice. This application consumes the greatest amount of calcium chloride. Calcium chloride is relatively harmless to plants and soil. As a de-icing agent, it is much more effective at lower temperatures than sodium chloride. When distributed for this use, it usually takes the form of small, white spheres a few millimeters in diameter, called prills. Solutions of calcium chloride can prevent freezing at temperatures as low as −52 °C (−62 °F), making it ideal for filling agricultural implement tires as a liquid ballast, aiding traction in cold climates.[14]
It is also used in domestic and industrial chemical air dehumidifiers.[15]
Road surfacing
[edit]
The second largest application of calcium chloride exploits its hygroscopic nature and the tackiness of its hydrates; calcium chloride is highly hygroscopic and its hydration is an exothermic process. A concentrated solution keeps a liquid layer on the surface of dirt roads, which suppresses the formation of dust. It keeps the finer dust particles on the road, providing a cushioning layer. If these are allowed to blow away, the large aggregate begins to shift around and the road breaks down. Using calcium chloride reduces the need for grading by as much as 50% and the need for fill-in materials as much as 80%.[16]
Food
[edit]In the food industry, calcium chloride is frequently employed as a firming agent in canned vegetables, particularly for canned tomatoes and cucumber pickles.[17][18][19][20] It is also used in firming soybean curds into tofu and in producing a caviar substitute from vegetable or fruit juices.[21][22][23] It is also used to enhance the texture of various other products, such as whole apples, whole hot peppers, whole and sliced strawberries, diced tomatoes, and whole peaches.[24][25]
The firming effect of calcium chloride can be attributed to several mechanisms:[24]
- Complexation, since calcium ions form complexes with pectin, a polysaccharide found in the cell wall and middle lamella of plant tissues.[24]
- Membrane stabilization, since calcium ions contribute to the stabilization of the cell membrane.[24]
- Turgor pressure regulation, since calcium ions influence cell turgor pressure, which is the pressure exerted by the cell contents against the cell wall.[24]
Calcium chloride's freezing-point depression properties are used to slow the freezing of the caramel in caramel-filled chocolate bars.[citation needed] Also, it is frequently added to sliced apples to maintain texture.[26]
In brewing beer, calcium chloride is sometimes used to correct mineral deficiencies in the brewing water. It affects flavor and chemical reactions during the brewing process, and can also affect yeast function during fermentation.[27][28][29][30][31]
In cheesemaking, calcium chloride is sometimes added to processed (pasteurized/homogenized) milk to restore the natural balance between calcium and protein in casein. It is added before the coagulant.[32]
Calcium chloride is also commonly used as an "electrolyte" in sports drinks and other beverages; as a food additive used in conjunction with other inorganic salts it adds taste to bottled water.[33][34][35]
The average intake of calcium chloride as food additives has been estimated to be 160–345 mg/day.[36] Calcium chloride is permitted as a food additive in the European Union for use as a sequestrant and firming agent with the E number E509.[37] It is considered as generally recognized as safe (GRAS) by the U.S. Food and Drug Administration.[38] Its use in organic crop production is generally prohibited under the US National Organic Program.[39]
The elemental calcium content in calcium chloride hexahydrate (CaCl2·6H2O) is approximately 18.2%. This means that for every gram of calcium chloride hexahydrate, there are about 182 milligrams of elemental calcium.
For anhydrous calcium chloride (CaCl2), the elemental calcium content is slightly higher, around 36.1% (for every gram of anhydrous calcium chloride there are about 361 milligrams of elemental calcium).
Calcium chloride has a very salty taste and can cause mouth and throat irritation at high concentrations, so it is typically not the first choice for long-term oral supplementation (as a calcium supplement).[40][41] Calcium chloride, characterized by its low molecular weight and high water solubility, readily breaks down into calcium and chloride ions when exposed to water. These ions are efficiently absorbed from the intestine.[42] However, caution should be exercised when handling calcium chloride, for it has the potential to release heat energy upon dissolution in water. This release of heat can lead to trauma and burns in the mouth, throat, esophagus, and stomach. In fact, there have been reported cases of stomach necrosis resulting from burns caused by accidental ingestions of big amounts of undissolved calcium chloride.[43][44]
The extremely salty taste of calcium chloride is used to flavor pickles without increasing the food's sodium content.[45]
Calcium chloride is used to prevent cork spot and bitter pit on apples by spraying on the tree during the late growing season.[46]
Laboratory and related drying operations
[edit]Drying tubes are frequently packed with calcium chloride. Kelp is dried with calcium chloride for use in producing sodium carbonate. Anhydrous calcium chloride has been approved by the FDA as a packaging aid to ensure dryness (CPG 7117.02).[47]
The hydrated salt can be dried for re-use but will dissolve in its own water of hydration if heated quickly and form a hard amalgamated solid when cooled.
Metal reduction flux
[edit]Similarly, CaCl2 is used as a flux and electrolyte in the FFC Cambridge electrolysis process for titanium production, where it ensures the proper exchange of calcium and oxygen ions between the electrodes.
Medical use
[edit]Calcium chloride infusions may be used as an intravenous therapy to prevent hypocalcemia.[48][49][50][51][52]
Calcium chloride is a highly soluble calcium salt. Hexahydrate calcium chloride (CaCl2·6H2O) has solubility in water of 811 g/L at 25 °C.[1] Calcium chloride when taken orally completely dissociates into calcium ions (Ca2+) in the gastrointestinal tract, resulting in readily bioavailable calcium. The high concentration of calcium ions facilitates efficient absorption in the small intestine.[42][53] However, the use of calcium chloride as a source of calcium taken orally is less common compared to other calcium salts because of potential adverse effects such as gastrointestinal irritation and discomfort.[53][54][55]
When tasted, calcium chloride exhibits a distinctive bitter flavor alongside its salty taste. The bitterness is attributable to the calcium ions and their interaction with human taste receptors: certain members of the TAS2R family of bitter taste receptors respond to calcium ions; the bitter perception of calcium is thought to be a protective mechanism to avoid ingestion of toxic substances, as many poisonous compounds taste bitter. While chloride ions (Cl⁻) primarily contribute to saltiness, at higher concentrations, they can enhance the bitter sensation. The combination of calcium and chloride ions intensifies the overall bitterness. At lower concentrations, calcium chloride may taste predominantly salty. The salty taste arises from the electrolyte nature of the compound, similar to sodium chloride (table salt). As the concentration increases, the bitter taste becomes more pronounced: the increased presence of calcium ions enhances the activation of bitterness receptors.[56][57][58]
Other applications
[edit]This section needs additional citations for verification. (May 2020) |
Calcium chloride is used in concrete mixes to accelerate the initial setting, but chloride ions lead to corrosion of steel rebar, so it should not be used in reinforced concrete.[59] The anhydrous form of calcium chloride may also be used for this purpose and can provide a measure of the moisture in concrete.[60]
Calcium chloride is included as an additive in plastics and in fire extinguishers, in blast furnaces as an additive to control scaffolding (clumping and adhesion of materials that prevent the furnace charge from descending), and in fabric softener as a thinner.[citation needed]
The exothermic dissolution of calcium chloride is used in self-heating cans and heating pads.[citation needed]
Calcium chloride is used as a water hardener in the maintenance of hot tub water, as insufficiently hard water can lead to corrosion and foaming.[citation needed]
In the oil industry, calcium chloride is used to increase the density of solids-free brines. It is also used to provide inhibition of swelling clays in the water phase of invert emulsion drilling fluids.[citation needed]
Calcium chloride (CaCl
2) acts as flux material, decreasing the melting point, in the Davy process for the industrial production of sodium metal through the electrolysis of molten NaCl.[citation needed]
Calcium chloride is also used in the production of activated charcoal.[citation needed]
Calcium chloride can be used to precipitate fluoride ions from water as insoluble CaF
2.[citation needed]
Calcium chloride is also an ingredient used in ceramic slipware. It suspends clay particles so that they float within the solution, making it easier to use in a variety of slipcasting techniques.[citation needed]
For watering plants to use as a fertilizer, a moderate concentration of calcium chloride is used to avoid potential toxicity: 5 to 10 mM (millimolar) is generally effective and safe for most plants—that is 0.55–1.11 grams (0.019–0.039 oz) of anhydrous calcium chloride (CaCl
2) per liter of water or 1.10–2.19 grams (0.039–0.077 oz) of calcium chloride hexahydrate (CaCl
2·6H
2O) per liter of water.[61][62] Calcium chloride solution is used immediately after preparation to prevent potential alterations in its chemical composition.[63][64] Besides that, calcium chloride is highly hygroscopic, meaning it readily absorbs moisture from the air.[65] If the solution is left standing, it can absorb additional water vapor, leading to dilution and a decrease in the intended concentration.[65] Prolonged standing may lead to the precipitation of calcium hydroxide or other insoluble calcium compounds, reducing the availability of calcium ions in the solution[66] and reducing the effectiveness of the solution as a calcium source for plants.[66] Nutrient solutions can become a medium for microbial growth if stored for extended periods.[67] Microbial contamination may alter the composition of the solution and potentially introduce pathogens to the plants.[67] When dissolved in water, calcium chloride can undergo hydrolysis, especially over time, which can lead to the formation of small amounts of hydrochloric acid and calcium hydroxide: Ca+
2+2H
2O ⇌ Ca(OH)
2+2H+
. This reaction can lower the pH of the solution, making it more acidic.[68] Acidic solutions may harm plant tissues and disrupt nutrient uptake.[69]
Calcium chloride dihydrate (20 percent by weight) dissolved in ethanol (95 percent ABV) has been used as a sterilant for male animals. The solution is injected into the testes of the animal. Within one month, necrosis of testicular tissue results in sterilization.[70][71][non-primary sources needed]
Cocaine producers in Colombia import tons of calcium chloride to recover solvents that are on the INCB Red List and are more tightly controlled.[72]
Hazards
[edit]Although the salt is non-toxic in small quantities when wet, the strongly hygroscopic properties of non-hydrated calcium chloride present some hazards. It can act as an irritant by desiccating moist skin. Solid calcium chloride dissolves exothermically, and burns can result in the mouth and esophagus if it is ingested. Ingestion of concentrated solutions or solid products may cause gastrointestinal irritation or ulceration.[73]
Consumption of calcium chloride can lead to hypercalcemia.[74]
Properties
[edit]
Calcium chloride dissolves in water, producing chloride and the aquo complex [Ca(H2O)6]2+. In this way, these solutions are sources of "free" calcium and free chloride ions. This description is illustrated by the fact that these solutions react with phosphate sources to give a solid precipitate of calcium phosphate:
- 3 CaCl2 + 2 PO3−4 → Ca3(PO4)2 + 6 Cl−
Calcium chloride has a very high enthalpy change of solution, indicated by considerable temperature rise accompanying dissolution of the anhydrous salt in water. This property is the basis for its largest-scale application.
Aqueous solutions of calcium chloride tend to be slightly acidic due to the influence of the chloride ions on the hydrogen ion concentration in water. The slight acidity of calcium chloride solutions is primarily due to the increased ionic strength of the solution, which can influence the activity of hydrogen ions and lower the pH slightly. The pH of calcium chloride in aqueous solution is the following:[75][76]
| Concentration (mol/L) | Approximate pH |
|---|---|
| 0.01 | 6.5 – 7.0 |
| 0.1 | 6.0 – 6.5 |
| 1.0 | 5.5 – 6.0 |
Molten calcium chloride can be electrolysed to give calcium metal and chlorine gas:
- CaCl2 → Ca + Cl2
Preparation
[edit]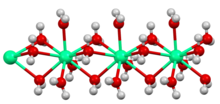
In much of the world, calcium chloride is derived from limestone as a by-product of the Solvay process, which follows the net reaction below:[10]
- 2 NaCl + CaCO3 → Na2CO3 + CaCl2
North American consumption in 2002 was 1,529,000 tonnes (3.37 billion pounds).[77] In the US, most calcium chloride is obtained by purification from brine. As with most bulk commodity salt products, trace amounts of other cations from the alkali metals and alkaline earth metals (groups 1 and 2) and other anions from the halogens (group 17) typically occur.[10]
Occurrence
[edit]Calcium chloride occurs as the rare evaporite minerals sinjarite (dihydrate) and antarcticite (hexahydrate).[78][79][80] Another natural hydrate known is ghiaraite – a tetrahydrate.[81][80] The related minerals chlorocalcite (potassium calcium chloride, KCaCl3) and tachyhydrite (calcium magnesium chloride, Ca Mg2Cl6·12H2O) are also very rare.[82][83][80] The same is true for rorisite, CaClF (calcium chloride fluoride).[84][80]
See also
[edit]References
[edit]- ^ a b c d e f g h i j Lide DR, ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ "Calcium chloride (anhydrous)". ICSC. International Programme on Chemical Safety and the European Commission. Archived from the original on 25 September 2015. Retrieved 18 September 2015.
- ^ Seidell A, Linke WF (1919). Solubilities of Inorganic and Organic Compounds (second ed.). New York: D. Van Nostrand Company. p. 196.
- ^ a b c d e f Anatolievich KR. "Properties of substance: calcium chloride". chemister.ru. Archived from the original on 24 June 2015. Retrieved 7 July 2014.
- ^ a b c d e f Pradyot P (2019). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. p. 162. ISBN 978-0-07-049439-8.
- ^ a b c d Müller U (2006). Inorganic Structural Chemistry (second ed.). England: John Wiley & Sons Ltd. p. 33. ISBN 978-0-470-01864-4.
- ^ a b c Sigma-Aldrich Co., Calcium chloride.
- ^ Garrett DE (2004). Handbook of Lithium and Natural Calcium Chloride. Elsevier. p. 379. ISBN 978-0-08-047290-4. Archived from the original on 31 October 2023. Retrieved 29 August 2018.
Its toxicity upon ingestion, is indicated by the test on rats: oral LD50 (rat) is 1.0–1.4 g/kg (the lethal dose for half of the test animals, in this case rats...)
- ^ "MSDS of Calcium chloride". fishersci.ca. Fisher Scientific. Archived from the original on 25 September 2015. Retrieved 7 July 2014.
- ^ a b c Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_547
- ^ Peck EL, Hamilton JH, Lewis JR, Hogan MB, Kusian RN, Cope WJ (1954). Proceedings of the First Annual Heating and Air Conditioning Conference: 1953-1955. University of Utah, Department of Metallurgy. Archived from the original on 15 March 2024. Retrieved 4 February 2024.
- ^ a b Hartmann PK (1816). Pharmacologia Dynamica: Usui Academico Adcommodata (in Latin). Kupffer et Wimmer. Archived from the original on 29 December 2023. Retrieved 29 December 2023.
- ^ Ottley WC (1826). A dictionary of chemistry and of mineralogy as connected with it. Murray. Archived from the original on 29 December 2023. Retrieved 29 December 2023.
- ^ "Binary Phase diagram: The Calcium Chloride – water system". Aqueous Solutions Aps. October 2016. Archived from the original on 26 June 2019. Retrieved 20 April 2017.
- ^ "Keeping Things Dry". humantouchofchemistry.com. Archived from the original on 26 October 2014. Retrieved 23 October 2014.
- ^ "Dust: Don't Eat It! Control It!". Road Management & Engineering Journal. US Roads (TranSafety Inc.). 1 June 1998. Archived from the original on 29 October 2007. Retrieved 9 August 2006.
- ^ M. Shafiur Rahman, ed. (2007). Handbook of Food Preservation (PDF) (2nd ed.). CRC Press. ISBN 978-1-57444-606-7. Archived (PDF) from the original on 5 April 2023. Retrieved 17 November 2024.
- ^ McFeeters RF, Pérez-Díaz I (2010). "Fermentation of Cucumbers Brined with Calcium Chloride Instead of Sodium Chloride". Journal of Food Science. 75 (3): C291-6. doi:10.1111/j.1750-3841.2010.01558.x. PMID 20492282.
- ^ Food Chemicals Codex. The United States Pharmacopeial Convention. ISBN 978-1-936424-26-9.
- ^ Guillou A, Floros J, Cousin M (1992). "Calcium Chloride and Potassium Sorbate Reduce Sodium Chloride used during Natural Cucumber Fermentation and Storage". Journal of Food Science. 57 (6): 1364–1368. doi:10.1111/j.1365-2621.1992.tb06859.x.
- ^ Devanampriyan Rajan, Chitra Devi Venkatachalam, Mahalakshmi R. L. Sruthi, Shaikh Mohd Riyan (2024). Emulsification and Spherification. Structured Foods. CRC Press. ISBN 978-1-003-35544-1.
- ^ Vega C, Ubbink J, Linden Ev (13 August 2013). The Kitchen as Laboratory: Reflections on the Science of Food and Cooking. Columbia University Press. ISBN 978-0-231-15345-4.
- ^ This H (18 August 2008). Molecular Gastronomy: Exploring the Science of Flavor. Columbia University Press. ISBN 978-0-231-13313-5.
- ^ a b c d e Luna-Guzmán I, Barrett DM (May 2000). "Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes". Postharvest Biology and Technology. 19 (1): 61–72. doi:10.1016/S0925-5214(00)00079-X.
- ^ "Apple Caviar Technique". StarChefs Studio. StarChefs.com. April 2004. Archived from the original on 29 June 2022. Retrieved 9 August 2006.
- ^ Sitbon C, Paliyath G (2011). "Pre- and Postharvest Treatments Affecting Nutritional Quality". Comprehensive Biotechnology. Pre- and Postharvest Treatments Affecting Nutritional Quality. Academic Press. pp. 349–357. doi:10.1016/B978-0-08-088504-9.00275-0. ISBN 978-0-08-088504-9.
- ^ Palmer JJ, Kaminski C (2013). Water: A Comprehensive Guide for Brewers. Brewers Publications. ISBN 978-0-937381-99-1.
- ^ Briggs B, Boulton C, Brooke P (28 September 2004). Brewing: Science and Practice. Woodhead Publishing Ltd. ISBN 978-1-85573-490-6.
- ^ Eumann M (2006). "Water in brewing". Brewing. Water in brewing. pp. 183–207. doi:10.1533/9781845691738.183. ISBN 978-1-84569-003-8.
- ^ Lewis MJ, Young TW (2001). Brewing. Water for brewing. pp. 57–70. doi:10.1007/978-1-4615-0729-1_4. ISBN 978-0-306-47274-9.
- ^ Walstra P, Walstra P, Wouters JT, Geurts TJ (29 September 2005). Dairy Science and Technology, Second Edition. Taylor & Francis. ISBN 978-0-8247-2763-5.
- ^ Fox PF, Guinee TP, Cogan TM, McSweeney PL (2017). Fundamentals of Cheese Science. Springer US. doi:10.1007/978-1-4899-7681-9. ISBN 978-1-4899-7681-9.
- ^ "Lab Suppliers: Calcium chloride dihydrate extra pure, for table water FCC, E 509. CAS 10035-04-8, pH 4.5 - 8.5 (50 g/l, H₂O, 20 °C)".
- ^ "Why Your Bottled Water Contains Four Different Ingredients". 24 July 2014. Archived from the original on 8 February 2019. Retrieved 17 March 2024.
- ^ "Brands Of Bottled Water With Electrolytes (Confirmed By Lab Tests) - Water Purification Guide". Archived from the original on 24 October 2021. Retrieved 4 July 2024.
- ^ Calcium Chloride SIDS Initial Assessment Profile, UNEP Publications, SIAM 15, Boston, 22–25 October 2002, pp. 13–14.
- ^ "Current EU approved additives and their E Numbers". Food Standards Agency. Archived from the original on 22 April 2022. Retrieved 17 November 2024.
- ^ 21 CFR § 184.1193
- ^ 7 CFR § 205.602 Archived 29 April 2021 at the Wayback Machine
- ^ "Calcium Chloride: Indications, Side Effects, Warnings". Archived from the original on 17 February 2023. Retrieved 15 March 2024.
- ^ Bendich A (January 2001). "Calcium supplementation and iron status of females". Nutrition. 17 (1): 46–51. doi:10.1016/s0899-9007(00)00482-2. PMID 11165888.
- ^ a b "Calcium chloride (CaCl2): Human health tier II assessment" (PDF). 27 November 2014. Archived (PDF) from the original on 16 March 2024. Retrieved 16 March 2024.
- ^ Remes-Troche JM (2 January 2013). "A 'black stomach' due to ingestion of anhydrous calcium chloride". BMJ Case Reports: bcr2012007716. doi:10.1136/bcr-2012-007716. PMC 3604345. PMID 23283618.
- ^ Nakagawa Y, Maeda A, Takahashi T, Kaneoka Y (2020). "Gastric Necrosis because of Ingestion of Calcium Chloride". ACG Case Reports Journal. 7 (8): e00446. doi:10.14309/crj.0000000000000446. PMC 7447462. PMID 32903978.
- ^ McMurtrie EK, Johanningsmeier SD (2018). "Quality of Cucumbers Commercially Fermented in Calcium Chloride Brine without Sodium Salts". Journal of Food Quality: 1–13. doi:10.1155/2018/8051435. S2CID 54004105.
- ^ "Cork Spot and Bitter Pit of Apples", Richard C. Funt and Michael A. Ellis, Ohioline.osu.edu/factsheet/plpath-fru-01
- ^ "CPG 7117.02". FDA Compliance Articles. US Food and Drug Administration. March 1995. Archived from the original on 13 December 2007. Retrieved 3 December 2007.
- ^ Loscalzo J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Larry Jameson J (2022). Harrison's Principles of Internal Medicine. McGraw Hill. ISBN 978-1-264-26850-4.
- ^ Hofer AM, Brown EM (2003). "Extracellular calcium sensing and signalling". Nature Reviews Molecular Cell Biology. 4 (7): 530–538. doi:10.1038/nrm1154. PMID 12838336.
- ^ Amir M, Sinha V, Kistangari G, Lansang MC (2020). "Clinical Characteristics of Patients with Type 2 Diabetes Mellitus Continued on Oral Antidiabetes Medications in the Hospital". Endocrine Practice. 26 (2): 167–173. doi:10.4158/EP-2018-0524. PMID 31557075.
- ^ Feingold KR, et al. (2000). Hypocalcemia: Diagnosis and Treatment. PMID 25905251.
- ^ Brunton LL, Knollmann BC (2022). Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw Hill. ISBN 978-1-264-25807-9.
- ^ a b Heaney RP, Recker RR, Weaver CM (May 1990). "Absorbability of calcium sources: the limited role of solubility". Calcif Tissue Int. 46 (5): 300–4. doi:10.1007/BF02563819. PMID 2110852.
- ^ Straub DA (June 2007). "Calcium supplementation in clinical practice: a review of forms, doses, and indications". Nutr Clin Pract. 22 (3): 286–96. doi:10.1177/0115426507022003286. PMID 17507729.
- ^ Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Ross AC, Taylor CL, Yaktine AL, Del Valle HB (2011). Dietary Reference Intakes for Calcium and Vitamin D. doi:10.17226/13050. ISBN 978-0-309-16394-1. PMID 21796828.
- ^ Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (November 2006). "The receptors and cells for mammalian taste". Nature. 444 (7117): 288–94. Bibcode:2006Natur.444..288C. doi:10.1038/nature05401. PMID 17108952.
- ^ Michael G. Tordoff (10 January 2001). "Calcium: Taste, Intake, and Appetite". Physiological Reviews. 81 (4): 1567–1597. doi:10.1152/physrev.2001.81.4.1567. PMID 11581497.
- ^ Breslin PA, Beauchamp GK (June 1997). "Salt enhances flavour by suppressing bitterness". Nature. 387 (6633): 563. Bibcode:1997Natur.387..563B. doi:10.1038/42388. PMID 9177340.
- ^ "Accelerating Concrete Set Time". Federal Highway Administration. 1 June 1999. Archived from the original on 17 January 2007. Retrieved 16 January 2007.
- ^ National Research Council (U.S.). Building Research Institute (1962). Adhesives in Building: Selection and Field Application; Pressure-sensitive Tapes. National Academy of Science-National Research Council. pp. 24–5.
- ^ Xu C, Li X, Zhang L (2 July 2013). "The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions". PLOS ONE. 8 (7): e68214. Bibcode:2013PLoSO...868214X. doi:10.1371/journal.pone.0068214. PMC 3699550. PMID 23844172.
- ^ Kang J, Zhao W, Zheng Y, Zhang DM, Zhou H, Sun P (13 April 2017). "Calcium chloride improves photosynthesis and water status in the C4 succulent xerophyte Haloxylon ammodendron under water deficit". Plant Growth Regulation. 82 (3): 467–478. doi:10.1007/s10725-017-0273-4.
- ^ Hepler PK (2005). "Calcium: A Central Regulator of Plant Growth and Development". The Plant Cell. 17 (8): 2142–2155. Bibcode:2005PlanC..17.2142H. doi:10.1105/tpc.105.032508. PMC 1182479. PMID 16024507.
- ^ Taiz L, Zeiger E (2015). Plant Physiology and Development (6th ed.). Sinauer Associates. pp. 157–159. ISBN 978-1-60535-255-8.
- ^ a b Perry DL, Phillips SL (2016). "Handbook of Inorganic Compounds". CRC Press: 98–99. ISBN 978-1-4398-1462-8.
- ^ a b Marschner P (2012). Marschner's Mineral Nutrition of Higher Plants (3rd ed.). Academic Press. pp. 135–137. ISBN 978-0-12-384905-2.
- ^ a b Jiao W, Zhou W, Tan D (2019). "Effects of Calcium Chloride on Microbial Community and Function in Rhizosphere Soil of Tomato Plants". Frontiers in Microbiology. 10 (2): 250–259. doi:10.3389/fmicb.2019.02052. PMC 6738277. PMID 31543892.
- ^ White PJ, Broadley MR (2003). "Calcium in Plants". Annals of Botany. 92 (4): 487–511. doi:10.1093/aob/mcg164. PMC 4243668. PMID 12933363.
- ^ Kirkby EA (1981). "Functional Aspects of Minerals in Plant Metabolism". Encyclopedia of Plant Physiology. 15: 679–698. doi:10.1007/978-3-642-68090-8_30 (inactive 24 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Koger LM (November 1977). "Calcium Chloride, Practical Necrotising Agent". The Bovine Practitioner: 118–119. doi:10.21423/bovine-vol1977no12p118-119 (inactive 3 December 2024).
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link)[non-primary source needed] - ^ Jana K, Samanta PK (2011). "Clinical Evaluation of Non-surgical Sterilization of Male Cats with Single Intra-testicular Injection of Calcium Chloride". BMC Veterinary Research. 7 (1): 39. doi:10.1186/1746-6148-7-39. PMC 3152893. PMID 21774835.[non-primary source needed]
- ^ Smith M, Simpson C (26 October 2020). "Narcos Are Waging a New Drug War Over a Texas Company's Basic Chemical". Bloomberg. Archived from the original on 26 October 2020. Retrieved 24 November 2024.
- ^ "Product Safety Assessment (PSA): Calcium Chloride". Dow Chemical Company. 2 May 2006. Archived from the original on 17 September 2009. Retrieved 22 July 2008.
- ^ "Calcium Chloride Possible Side Affects". www.drugs.com. Archived from the original on 27 July 2020. Retrieved 23 January 2018.
- ^ Speight J (5 October 2016). Lange's Handbook of Chemistry, Seventeenth Edition. McGraw-Hill Education. ISBN 978-1-259-58609-5.
- ^ Rumble JR (4 June 2024). CRC Handbook of Chemistry and Physics. CRC Press. ISBN 978-1-032-65562-8.
- ^ Calcium Chloride SIDS Initial Assessment Profile, UNEP Publications, SIAM 15, Boston, 22–25 October 2002, page 11.
- ^ "Sinjarite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 6 November 2020.
- ^ "Antarcticite". www.mindat.org. Archived from the original on 1 May 2023. Retrieved 6 November 2020.
- ^ a b c d "List of Minerals". www.ima-mineralogy.org. 21 March 2011. Archived from the original on 15 March 2013. Retrieved 6 November 2020.
- ^ "Ghiaraite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 6 November 2020.
- ^ "Chlorocalcite". www.mindat.org. Archived from the original on 30 May 2023. Retrieved 6 November 2020.
- ^ "Tachyhydrite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 6 November 2020.
- ^ "Rorisite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 6 November 2020.
- Greenwood NN, Earnshaw A (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
External links
[edit]- International Chemical Safety Card 1184
- Product and Application Information (Formerly Dow Chemical Calcium Chloride division) Archived 17 September 2023 at the Wayback Machine
- Report on steel corrosion by chloride including CaCl2 Archived 16 June 2011 at the Wayback Machine
- Collection of calcium chloride reports and articles
- Calcium chloride, Anhydrous MSDS
- Difusivity of calcium chloride
- Centers for Disease Control and Prevention, National Institutes of Occupational Safety and Health, "Calcium Chloride (anhydrous)"

